Our technology
Even in times of modern medicine, cancer is a fundamental turning point in a person's life. Every year 10 million people die of cancer worldwide; in Germany, statistically, a cancer patient dies about every 2 minutes.
First, diagnostics differentiates down to the genetic level to which type of cancer the disease can be assigned. However, in most cases one of the three major therapeutic measures is still used:
chemotherapy, surgery and radiation. It is often not possible to respond specifically to the cause of the cancer because the appropriate therapeutic agents are missing. The development and research of new drugs still takes a long time.
Over 10 years ago, Drs. Nicole and Sebastian Bäumer began researching RNA interference as a therapeutic principle against cancer in the laboratory of Prof. Wolfgang Berdel's department. RNA interference uses a natural mechanism that every single cell harbors to destroy the mRNA of any factor in the cell. Then, for example, a cancer-driving protein can no longer be formed from this mRNA and the cancer cell dies. This process was considered so important that Andrew Fire and Craig Mello were awarded the Nobel Prize in Medicine in 2006 for the discovery of this mechanism. But so far, there are only a handful of approved drugs that use RNA interference as their active principle.
Why is that?
This method has its pitfalls: The inhibitory so-called small inhibitory RNA or siRNA is quickly degraded in the blood. And even if it gets to the intented cancer cell, this cell does not voluntarily take up siRNA, it is left out.
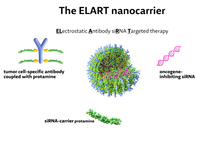
These problems are solved with our proprietary technology, the ELART platform: The natural protein protamine electrostatically binds the siRNA and thus protects it from degradation. An antibody that recognizes a surface molecule on the cancer cell is also coupled with protamine and the mixture of protamine, siRNA and antibody-protamine voluntarily forms a spherical nanocarrier that safely transports the siRNA to the cancer cell:
Our research
Until now, the therapeutic use of RNAi has always been compromised by the missing delivery of the active substance siRNA to the target cell. For this purpose, we have developed a principle of tumor cell-specific application of siRNAs using target cell-specific surface receptor antibodies. The siRNAs are directed against highly specific oncogenes, and a combination of different siRNAs is also applicable. Here, the use of an oncogene-specific siRNA and the choice of a tumor-specific surface receptor offers a double level of specificity and focuses the siRNA treatment on the tumor cells. The universal carrier protamine has been clinically used for decades and can be used in all applications. We developed the principle at our scientific laboratories situated at the University Hospital Münster, Dept. of Medicine A.
Our Publications
The experimental and preclinical data for the nanocarriers have been published in multiple high-ranking, peer-reviewed articles: